How to Test Dietary Fiber for Nutrition Facts Panels

Many of our clients are ordering dietary fiber tests to meet the FDA changes to nutrition labels in 2020. If your company is wondering how to best test for dietary fiber, this blog can help you determine which dietary fiber tests you may request from Medallion Labs.
What is dietary fiber?
The Nutrition Facts Label final rule defines dietary fiber as:
Non-digestible soluble and insoluble carbohydrates (with 3 or more monomeric units), and lignin that are intrinsic and intact in plants; isolated or synthetic non-digestible carbohydrates (with 3 or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health.
How do you determine which dietary fibers may be declared on Nutrition Facts Panels?
Dietary fiber that may be declared on Nutrition Facts Panels includes most naturally occurring fibers that are intrinsic and intact in plants.
In addition, added isolated or synthetic non-digestible soluble and insoluble carbohydrates that FDA has determined to have beneficial physiological effects to human health may be declared. These beneficial effects include lowering blood glucose and cholesterol levels, reducing calorie intake, and increasing the frequency of bowel movements.
Nutrition Labeling
Our testing services can help you ensure regulatory compliance for nutritional labeling.
Learn moreHow do you choose the appropriate dietary fiber testing methodology?
FDA recommends starting with Codex Fiber methods for nutrition facts panel and nutritional labeling purposes.
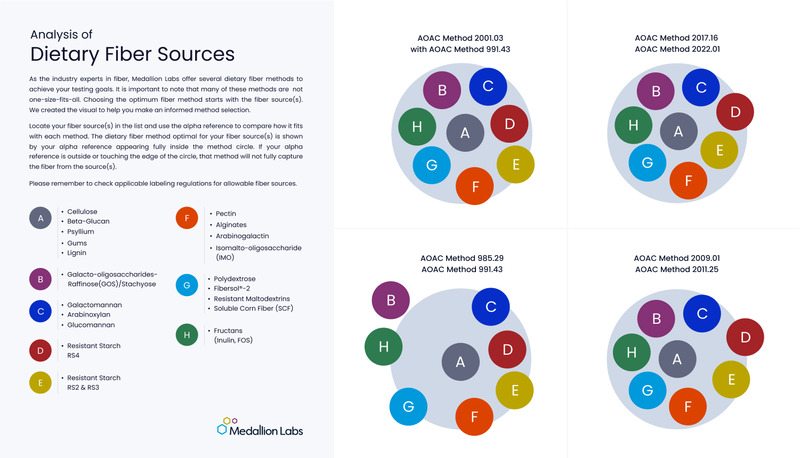
AOAC 2011.25 is the optimal method to accurately capture dietary fiber within any one ingredient or finished product as the enzymatic digestion portion of the method most closely mimics human physiological digestion. This method quantifies both soluble and insoluble dietary fiber and reports the sum of the two as total dietary fiber.
AOAC 2009.01 uses the same digestion as the AOAC 2011.25 method but the soluble and insoluble portions are not separated, thus AOAC 2009.01 reports only total dietary fiber.
AOAC 991.43 - Fiber (Total – Gravimetric) or AOAC 991.43 - Fiber (Insoluble, Soluble, Total – Gravimetric) may be suitable for your sample depending on the composition and can either quantify both soluble and insoluble dietary fiber and report the sum of the two as total dietary fiber, or report only total dietary fiber.
See the table below for Medallion Labs’ recommendations on which fiber test to choose based on FDA approved fiber source.
| FDA Approved Fiber Source | Total Fiber Method | Insoluble, Soluble, Total Fiber Method |
| Beta-glucan soluble fiber | 991.43 | 991.43 |
| Psyllium husk | 991.43 | 991.43 |
| Cellulose | 991.43 | 991.43 |
| Guar gum | 991.43 | 991.43 |
| Pectin | 2009.01 | 2011.25 |
| Locust bean gum | 991.43 | 991.43 |
| Hydroxypropylmethylcellulose | Contact Us | Contact Us |
| Mixed plant cell wall fibers (a broad category that includes fibers like sugar cane fiber and apple fiber, among many others) | 2009.01 | 2011.25 |
| Arabinoxylan | 2009.01 | 2011.25 |
| Alginate | 2009.01 | 2011.25 |
| Inulin and inulin-type fructans | 2009.01 | 2011.25 |
| High amylose starch (resistant starch 2) | 2009.01 | 2011.25 |
| Galactooligosaccharide | 2009.01 | 2011.25 |
| Polydextrose | 2009.01 | 2011.25 |
| Resistant maltodextrin/dextrin | 2009.01 | 2011.25 |
Are there fiber sources in my sample that Medallion Labs should know about prior to testing?
For samples containing any of the following ingredients or dietary fiber sources, it is important to indicate on your submission form that they are present; in addition, please state the levels of these sources, if you have them available.
It is important to let Medallion Labs know if your samples contain these ingredients so that we can correctly interpret the results of the chromatographic portion of the test.
- Psyllium
- Xanthan Gum
- Carrageenan
- Tapioca Fiber
- FiberSym
- Konjac
- Fenugreek
- Galactomannan
- Guar
- Acacia
- Hydroxypropylmethylcellulose (HPMC)
Does Medallion Labs test IMO?
While IMO is not currently considered a dietary fiber in the United States, several of our fiber methods will include IMO in the fiber result if the ingredient is present. Medallion Labs will not be able to adjust the fiber results to take into account the IMO content. If IMO ingredients are being used, adjustments may be needed for accurate labeling in the United States and we recommend companies to work with a food consultant or regulation expert.
How should we account for the additional dietary fibers that FDA intends to propose?
Until FDA completes rulemaking, regarding adding additional fibers to the regulatory definition of dietary fiber, the agency intends to exercise enforcement discretion.
This will allow manufacturers to include the amount of these additional fibers in the dietary fiber declaration on the Nutrition and Supplement Facts labels until rulemaking is completed.
How do manufacturers know how much dietary fiber to declare on the FDA Nutrition and Supplement Facts labels and how should this be documented?
The amount of dietary fibers declared should represent the total dietary fiber that is quantified by analytical methods minus the amount that does not meet the dietary fiber definition.
Analytical methods cannot distinguish between non-digestible carbohydrates that do and do not meet the dietary fiber definition. Therefore, companies must keep records for those foods that contain both non-digestible carbohydrates that do meet the regulatory definition of dietary fiber and added non-digestible carbohydrates that do not meet the definition of dietary fiber.
If a product contains added isolated or synthetic non-digestible carbohydrates that do not meet the definition of dietary fiber, what is the deadline for manufacturers to modify product labeling?
FDA has issued a final rule to extend the compliance date to January 1, 2020, for the Nutrition Facts and Supplement Facts label final rule and the Serving Size final rule for manufacturers with $10 million or more in annual food sales.
Manufacturers with less than $10 million in annual food sales have until January 1, 2021, to comply.
Ready to test for dietary fiber?
If your company is ready to test for dietary fiber, you may order tests from Medallion Labs today or contact us for more information about our testing services.
Let's Get to Work!
Submit your order online and ship your samples today. If you have questions, we are always here to help.
Medallion Labs+
A food testing program designed with mid-market and enterprise food and ingredient manufacturers in mind.